| 1 |
Isik S, Yeman Kiyak B, Akbayir R, et al. Microglia mediated neuroinflammation in Parkinson's disease[J]. Cells, 2023, 12 (7): 1012.
doi: 10.3390/cells12071012
|
| 2 |
Wang S, Jiang S, Wu J, et al. Trends in Parkinson's disease mortality in China from 2004 to 2021: a joinpoint analysis[J]. BMC Public Health, 2024, 24 (1): 1091.
doi: 10.1186/s12889-024-18532-8
|
| 3 |
Calabresi P, Mechelli A, Natale G, et al. Alpha-synuclein in Parkinson's disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction[J]. Cell Death Dis, 2023, 14 (3): 176.
doi: 10.1038/s41419-023-05672-9
|
| 4 |
Muleiro Alvarez M, Cano-Herrera G, Osorio Martínez MF, et al. A comprehensive approach to Parkinson's disease: addressing its molecular, clinical, and therapeutic aspects[J]. Int J Mol Sci, 2024, 25 (13): 7183.
doi: 10.3390/ijms25137183
|
| 5 |
祁羚, 李亚楠, 汪瑶, 等. 电针对帕金森病模型小鼠运动功能的影响及相关分子机制探讨[J]. 北京中医药大学学报, 2024, 47 (5): 721- 728.
|
| 6 |
Murakami H, Shiraishi T, Umehara T, et al. Recent advances in drug therapy for Parkinson's disease[J]. Intern Med, 2023, 62 (1): 33- 42.
doi: 10.2169/internalmedicine.8940-21
|
| 7 |
Bologna M, Guerra A, Colella D, et al. Objective assessment of the effects of opicapone in Parkinson's disease through kinematic analysis[J]. Neurol Sci, 2024, 45 (5): 2035- 2046.
doi: 10.1007/s10072-023-07233-6
|
| 8 |
Irmady K, Hale CR, Qadri R, et al. Blood transcriptomic signatures associated with molecular changes in the brain and clinical outcomes in Parkinson's disease[J]. Nat Commun, 2023, 14 (1): 3956.
doi: 10.1038/s41467-023-39652-6
|
| 9 |
Cappelletti C, Henriksen SP, Geut H, et al. Transcriptomic profiling of Parkinson's disease brains reveals disease stage specific gene expression changes[J]. Acta Neuropathol, 2023, 146 (2): 227- 244.
doi: 10.1007/s00401-023-02597-7
|
| 10 |
van den Hurk M, Lau S, Marchetto MC, et al. Druggable transcriptomic pathways revealed in Parkinson's patient-derived midbrain neurons[J]. NPJ Parkinsons Dis, 2022, 8 (1): 134.
doi: 10.1038/s41531-022-00400-0
|
| 11 |
聂嘉璇, 钱文秀, 王曼姝, 等. 中药毒性相关数据库的研究现状及对比研[J]. 中草药, 2023, 54 (22): 7588- 7596.
doi: 10.7501/j.issn.0253-2670.2023.22.035
|
| 12 |
Lim N, Pavlidis P. Evaluation of connectivity map shows limited reproducibility in drug repositioning[J]. Sci Rep, 2021, 11 (1): 17624.
doi: 10.1038/s41598-021-97005-z
|
| 13 |
Gao L, Zhao G, Fang JS, et al. Discovery of the neuroprotective effects of alvespimycin by computational prioritization of potential anti-Parkinson agents[J]. FEBS J, 2014, 281 (4): 1110- 1122.
doi: 10.1111/febs.12672
|
| 14 |
Fan LY, Yang J, Liu RY, et al. Integrating single-nucleus sequence profiling to reveal the transcriptional dynamics of Alzheimer's disease, Parkinson's disease, and multiple sclerosis[J]. J Transl Med, 2023, 21 (1): 649.
doi: 10.1186/s12967-023-04516-6
|
| 15 |
Fletcher EJR, Jamieson AD, Williams G, et al. Targeted repositioning identifies drugs that increase fibroblast growth factor 20 production and protect against 6-hydroxydopamine-induced nigral cell loss in rats[J]. Sci Rep, 2019, 9 (1): 8336.
doi: 10.1038/s41598-019-44803-1
|
| 16 |
Vargas DM, De Bastiani MA, Parsons RB, et al. Parkinson's disease master regulators on substantia nigra and frontal cortex and their use for drug repositioning[J]. Mol Neurobiol, 2021, 58 (4): 1517- 1534.
doi: 10.1007/s12035-020-02203-x
|
| 17 |
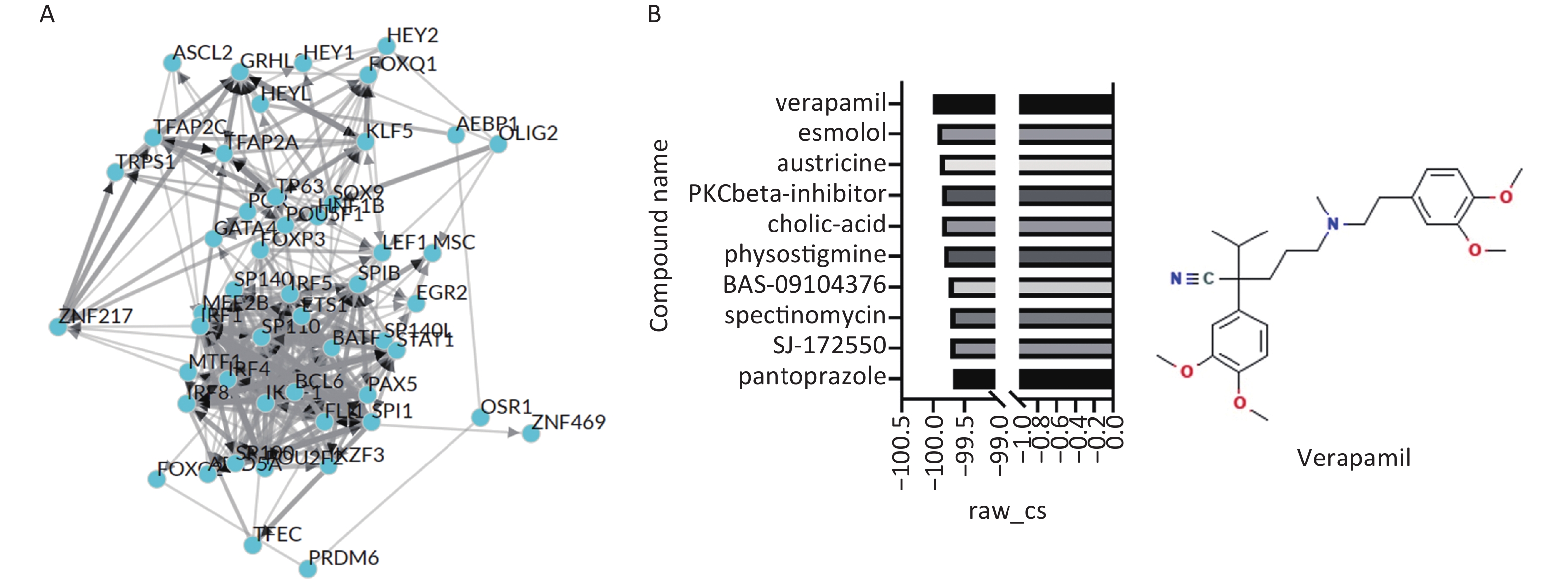
Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks[J]. Genome Res, 2003, 13 (11): 2498- 2504.
doi: 10.1101/gr.1239303
|
| 18 |
Keenan AB, Torre D, Lachmann A, et al. ChEA3: transcription factor enrichment analysis by orthogonal omics integration[J]. Nucleic Acids Res, 2019, 47 (W1): W212- W224.
doi: 10.1093/nar/gkz446
|
| 19 |
Lamb J, Crawford ED, Peck D, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease[J]. Science, 2006, 313 (5795): 1929- 1935.
doi: 10.1126/science.1132939
|
| 20 |
Kalia LV, Lang AE. Parkinson's disease[J]. Lancet, 2015, 386 (9996): 896- 912.
doi: 10.1016/S0140-6736(14)61393-3
|
| 21 |
Blazejewski SM, Bennison SA, Ha NT, et al. Rpsa signaling regulates cortical neuronal morphogenesis via its ligand, PEDF, and plasma membrane interaction partner, Itga6[J]. Cereb Cortex, 2022, 32 (4): 770- 795.
doi: 10.1093/cercor/bhab242
|
| 22 |
Ma S, Li X, Cao R, et al. Developmentally regulated expression of integrin alpha-6 distinguishes neural crest derivatives in the skin[J]. Front Cell Dev Biol, 2023, 11, 1140554.
doi: 10.3389/fcell.2023.1140554
|
| 23 |
Li Z, Zhang B, Yao W, et al. APC-Cdh1 regulates neuronal apoptosis through modulating glycolysis and pentose-phosphate pathway after oxygen-glucose deprivation and reperfusion[J]. Cell Mol Neurobiol, 2019, 39 (1): 123- 135.
doi: 10.1007/s10571-018-0638-x
|
| 24 |
Valdez-Sinon AN, Lai A, Shi L, et al. Cdh1-APC regulates protein synthesis and stress granules in neurons through an FMRP-dependent mechanism[J]. iScience, 2020, 23 (5): 101132.
doi: 10.1016/j.isci.2020.101132
|
| 25 |
Carriba P, Davies AM. CD40 is a major regulator of dendrite growth from developing excitatory and inhibitory neurons[J]. Elife, 2017, 6, e30442.
doi: 10.7554/eLife.30442
|
| 26 |
Ots HD, Tracz JA, Vinokuroff KE, et al. CD40-CD40L in neurological disease[J]. Int J Mol Sci, 2022, 23 (8): 4115.
|
| 27 |
Cheong RY, Czieselsky K, Porteous R, et al. Expression of ESR1 in glutamatergic and GABAergic neurons is essential for normal puberty onset, estrogen feedback, and fertility in female mice[J]. J Neurosci, 2015, 35 (43): 14533- 14543.
doi: 10.1523/JNEUROSCI.1776-15.2015
|
| 28 |
Chen G, Lai S, Jiang S, et al. Cellular and circuit architecture of the lateral septum for reward processing[J]. Neuron, 2024, 112 (16): 2783- 2798.
doi: 10.1016/j.neuron.2024.06.004
|
| 29 |
Zyuz'kov GN, Miroshnichenko LA, Polyakova TY, et al. The role of Smad3 in the realization of the growth potential of different types of neural progenitor cells and the secretory function of neuroglia[J]. Bull Exp Biol Med, 2024, 177 (1): 35- 38.
doi: 10.1007/s10517-024-06126-8
|
| 30 |
Hiew LF, Poon CH, You HZ, et al. TGF-β/Smad signalling in neurogenesis: Implications for neuropsychiatric diseases[J]. Cells, 2021, 10 (6): 1382.
doi: 10.3390/cells10061382
|
| 31 |
Shan Y, Farmer SM, Wray S. Drebrin regulates cytoskeleton dynamics in migrating neurons through interaction with CXCR4 [J]. Proc Natl Acad Sci U S A, 2021, 118(3): e2009493118.
|
| 32 |
Terheyden-Keighley D, Hilla AM, Fischer D. CXCR4 signaling in central nervous system regeneration: friend or foe ?[J]. Neural Regen Res, 2022, 17 (7): 1481- 1483.
doi: 10.4103/1673-5374.330605
|
| 33 |
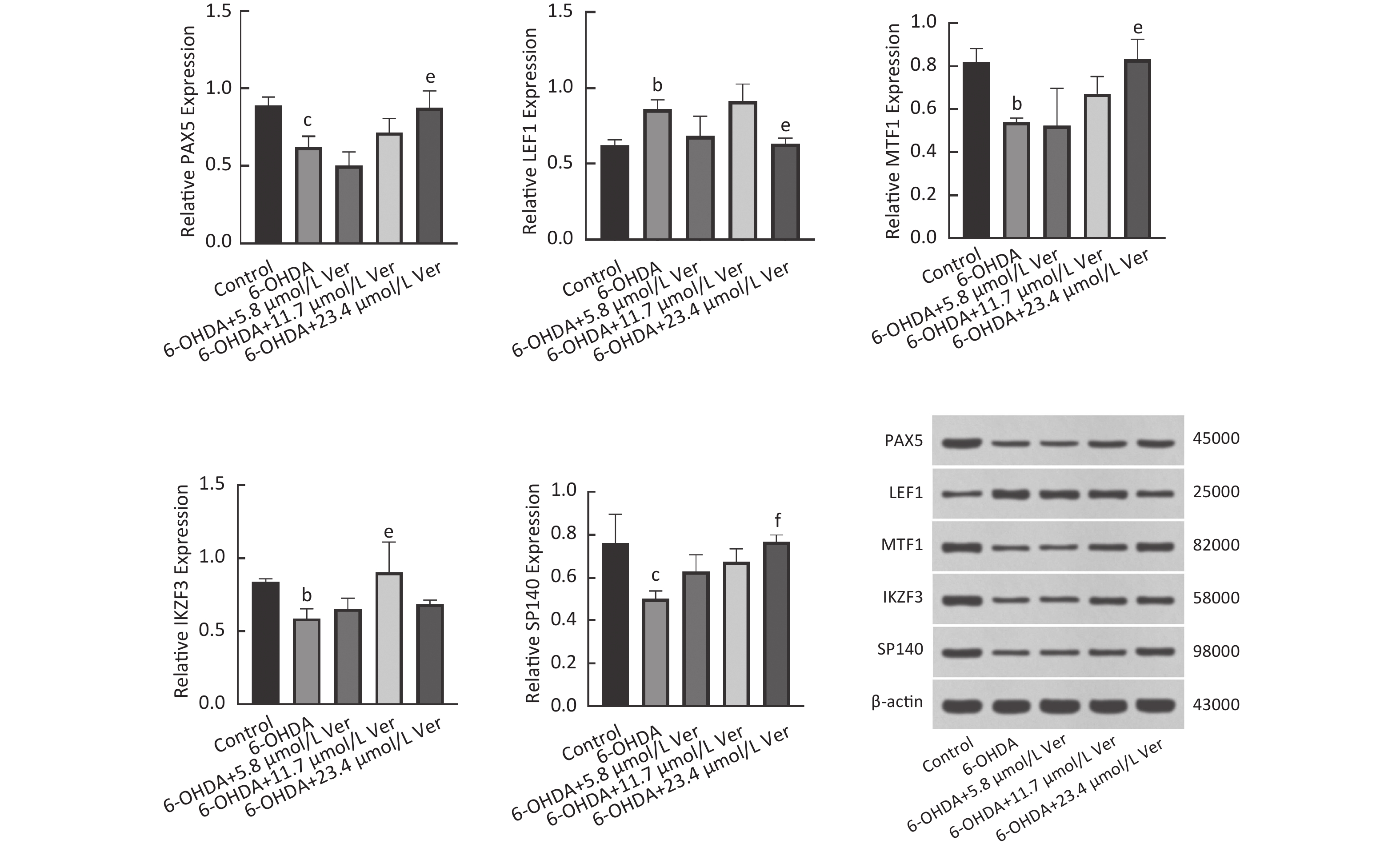
Maurya SK, Mishra R. Co-localization and interaction of Pax5 with Iba1 in brain of mice[J]. Cell Mol Neurobiol, 2018, 38 (4): 919- 927.
doi: 10.1007/s10571-017-0566-1
|
| 34 |
Ohtsuka N, Badurek S, Busslinger M, et al. GABAergic neurons regulate lateral ventricular development via transcription factor Pax5[J]. Genesis, 2013, 51 (4): 234- 245.
doi: 10.1002/dvg.22370
|
| 35 |
Santos R, Linker SB, Stern S, et al. Deficient LEF1 expression is associated with lithium resistance and hyperexcitability in neurons derived from bipolar disorder patients[J]. Mol Psychiatry, 2021, 26 (6): 2440- 2456.
doi: 10.1038/s41380-020-00981-3
|
| 36 |
Nouri P, Götz S, Rauser B, et al. Dose-dependent and subset-specific regulation of midbrain dopaminergic neuron differentiation by LEF1-Mediated WNT1/b-Catenin signaling[J]. Front Cell Dev Biol, 2020, 8, 587778.
doi: 10.3389/fcell.2020.587778
|
| 37 |
Valsecchi V, Laudati G, Cuomo O, et al. The hypoxia sensitive metal transcription factor MTF-1 activates NCX1 brain promoter and participates in remote postconditioning neuroprotection in stroke[J]. Cell Death Dis, 2021, 12 (5): 423.
doi: 10.1038/s41419-021-03705-9
|
| 38 |
Ferlazzo GM, Gambetta AM, Amato S, et al. Genome-wide screening in pluripotent cells identifies Mtf1 as a suppressor of mutant huntingtin toxicity[J]. Nat Commun, 2023, 14 (1): 3962.
doi: 10.1038/s41467-023-39552-9
|
| 39 |
Meng C, Chen S, He Q, et al. IKZF3 modulates cerebral ischemia/reperfusion injury by inhibiting neuroinflammation[J]. Int Immunopharmacol, 2023, 114, 109480.
doi: 10.1016/j.intimp.2022.109480
|
| 40 |
Fraschilla I, Jeffrey KL. The speckled protein (SP) family: Immunity's chromatin readers[J]. Trends Immunol, 2020, 41 (7): 572- 585.
doi: 10.1016/j.it.2020.04.007
|
| 41 |
Karaky M, Fedetz M, Potenciano V, et al. SP140 regulates the expression of immune-related genes associated with multiple sclerosis and other autoimmune diseases by NF-κB inhibition[J]. Hum Mol Genet, 2018, 27 (23): 4012- 4023.
doi: 10.1093/hmg/ddy284
|
| 42 |
Mokhtari M, Khoshbakht S, Akbari ME, et al. BMC3PM: bioinformatics multidrug combination protocol for personalized precision medicine and its application in cancer treatment[J]. BMC Med Genomics, 2023, 16 (1): 328.
doi: 10.1186/s12920-023-01745-y
|
| 43 |
Li Y, Gao S, Guo Z, et al. Screening of potential drugs for the treatment of diabetic kidney disease using single-cell transcriptome sequencing and connectivity map data[J]. Biochem Biophys Res Commun, 2024, 725, 150263.
doi: 10.1016/j.bbrc.2024.150263
|
| 44 |
Gao Y, Zhang J, Tang T, et al. Hypoxia pathways in Parkinson's disease: from pathogenesis to therapeutic targets[J]. Int J Mol Sci, 2024, 25 (19): 10484.
doi: 10.3390/ijms251910484
|
| 45 |
Fang W, Lv P, Geng X, et al. Penetration of verapamil across blood brain barrier following cerebral ischemia depending on both paracellular pathway and P-glycoprotein transportation[J]. Neurochem Int, 2013, 62 (1): 23- 30.
doi: 10.1016/j.neuint.2012.10.012
|
| 46 |
Li Y, Qiu L, Liu X, et al. PINK1 alleviates myocardial hypoxia-reoxygenation injury by ameliorating mitochondrial dysfunction[J]. Biochem Biophys Res Commun, 2017, 484 (1): 118- 124.
doi: 10.1016/j.bbrc.2017.01.061
|
| 47 |
叶玲, 叶娟, 鲁继光, 等. 共载多西他赛和维拉帕米脂质体逆转肿瘤耐药性的研究[J]. 药学学报, 2020, 55 (5): 1035- 1041.
doi: 10.16438/j.0513-4870.2019-0662
|
| 48 |
Yang T, Ferrill L, Gallant L, et al. Verapamil and riluzole cocktail liposomes overcome pharmacoresistance by inhibiting P-glycoprotein in brain endothelial and astrocyte cells: a potent approach to treat amyotrophic lateral sclerosis[J]. Eur J Pharm Sci, 2018, 120, 30- 39.
doi: 10.1016/j.ejps.2018.04.026
|
| 49 |
Lal R, Singh A, Watts S, et al. Experimental models of Parkinson's disease: Challenges and opportunities[J]. Eur J Pharmacol, 2024, 980, 176819.
doi: 10.1016/j.ejphar.2024.176819
|
| 50 |
胡毅龙, 赵怡楠, 苗晋鑫, 等. 大小鼠帕金森病行为学评价方法概述及常用动物模型特点分析[J]. 中国实验动物学报, 2024, 32 (7): 942- 954.
doi: 10.3969/j.issn.1005-4847.2024.07.013
|